Publications
-
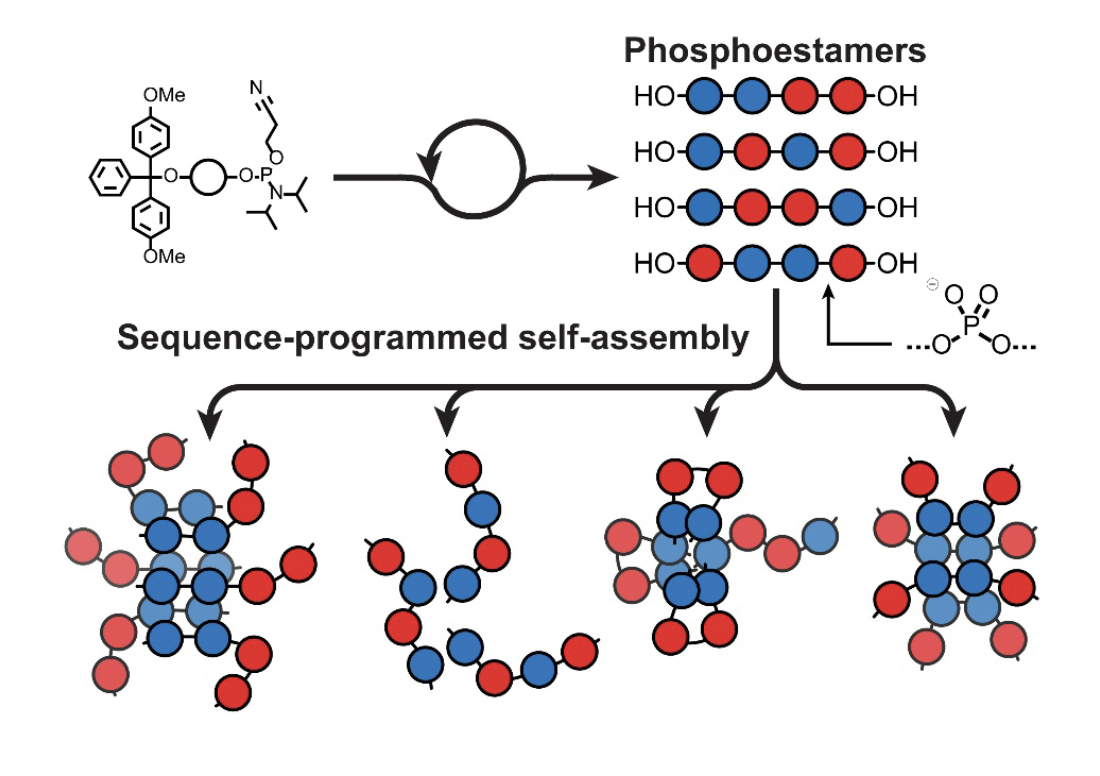
98. Differential Self-Assembly of Sequence-Isomeric Phosphoestamers
J. Williamson, T. Piskorz, B. Claringbold, A. P. N. Harvey, F. Duarte, C. Serpell. ChemRxiv. 2025.
-

97. Benchmark of double-ended transition state search methods for metal-catalysed reactions
S. R. Maiti, D. Buttar, F. Duarte. ChemRxiv 2025
-

96. Distillation of atomistic foundation models across architectures and chemical domains
J. L. A. Gardner, D. F. Thomas du Toit, C. B. Mahmoud, Z. F. Beaulieu, V. Juraskova, L. Paşca, L. A. M. Rosset, F. Duarte, F. Martelli, C. J. Pickard, V. L. Deringer. arXiv 2025
-

95. An exploration of dataset. bias in single-step retrosynthesis prediction
S. Tanovic, E. Wieczorek, F. Duarte. Dig. Discov. 2026 ASAP. ChemRxiv2025
-

94. Kinetic predictions for SN2 reactions using the BERT architecture: Comparison and interpretation
C. Wilson, M. Calvo, S. Zavitsanou, J. Somper, E. Wieczorek, T. Watts, J. Crain, F. Duarte, Dig. Discov. 2026 DOI: 10.1039/D5DD00192G. ChemRxiv2025
-

93. Collective asymmetric synthesis of the Strychnos alkaloids via thiophene S,S-dioxide cycloaddition cascades
K. H. K. Park, J. Park, N. Frank, H. Zhang, F. Duarte, E. A. Anderson. ChemRxiv 2024
-

92. Active learning meets metadynamics: Automated workflow for reactive machine learning potentials
Valdas Vitartas,‡ Hanwen Zhang,‡ Veronika Juraskova, Tristan Johnston-Wood, Fernanda Duarte.* Dig. Discov. 2026. DOI: 10.1039/D5DD00261C. ChemRxiv
-
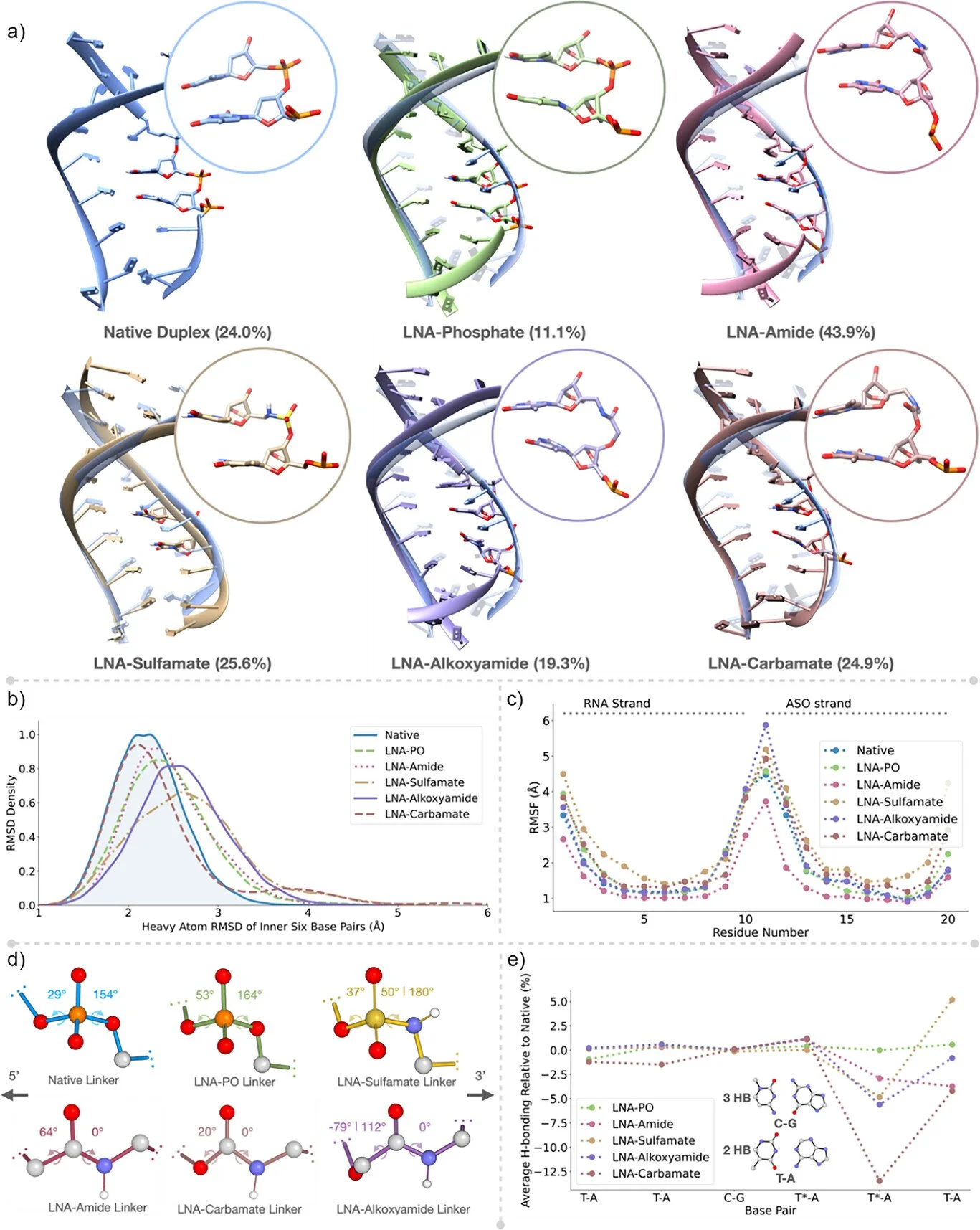
91. Synthesis, Biological Activity, and Molecular Dynamics Simulations of LNA‐Charge Neutral Linkages for Enhanced Splice‐Switching Antisense Oligonucleotides
A. Kennett, L. Lie, M. Flerin, B. Z. Kurt, Y. R. Baker, A. C. Hill, A. Ramesh, M. J.A. Wood, D. Dhara, A. H. El-Sagheer,* F. Duarte,* T. Brown,* Angew. Chem. Int. Ed.2025, 64, e202511386.
-
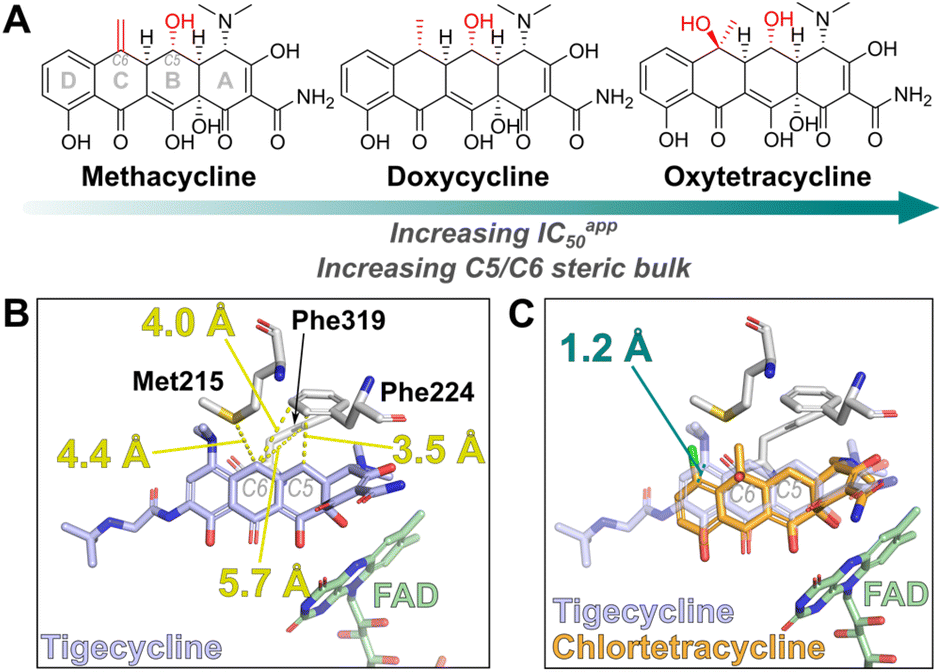
90. Binding assays enable discovery of Tet(X) inhibitors that combat tetracycline destructase resistance
M. J. Beech, E. C. Toma, H. G. Smith, M. M. Trush, J. H. J. Ang, M. Y. Wong, C. H. J. Wong, H. S. Ali, Z. Butt, V. Goel, F. Duarte, A. J. M. Farley, T. R. Walsh, C. J. Schofield. Chem. Sci. 2025, 16, 9691-9704.
-

89. RaPID discovery of cell-permeable helical peptide inhibitors con-taining cyclic β-amino acids against SARS-CoV-2 main protease
M. Kawai, T. R. Malla, H. T. H. Chan, A. Tumber, L. Brewitz, E. Salah, N. Terasaka, T. Katoh, A. Kawamura, C. J. Schofield, F. Duarte, H. Suga. RSC Chem. Biol.2025, 6, 1089-1099.
-
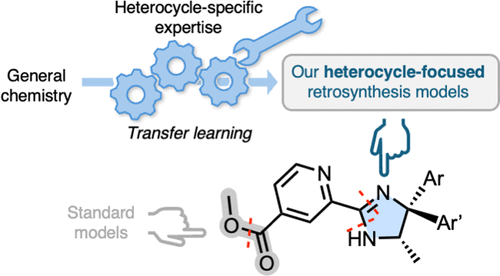
88. Transfer learning for Heterocycle Synthesis Prediction
E. Wieczorek, J. W. Sin, S. Tanovic, M. T. O. Holland, L. Wilbraham, V. S. Perez, A. Bradley, D. Miketa, P. E. Brennan, F. Duarte*, J. Chem. Inf. Model. 2025, 65, 15, 7851–7861
-

87. Dissecting the Effects of Cage Structure in the Catalytic Activation of Imide Chlorenium-Ion Donors
T. Zhou, T. K. Piskorz, K. Liu, Y. Lu, F. Duarte, P. J. Lusby. J. Am. Chem. Soc. 2025, 147 (13), 11456-11464.
-

86. Chloride Selective, Non-protonophoric Ion Transport with Macrocyclic Halogen Bonding Anionophores
M. Flerin, F. Duarte, M. Langton. Chem. Eur. J. 2025, 31, e202502033.
-

85. Dynamic Kinetic Resolution Allows Control of Remote Stereochemistry in Asymmetric Hydrogen Borrowing Alkylation
D. M. J. Cheang, J. L. Crompton, M. M. Amer, F. Battiti, B. B. Skjelstad, K. E. Christensen, P. Barton, F. Duarte, T. J. Donohoe. Angew. Chem. Int. Ed. 2025, 64, e202424959.
-

84. Modelling ligand exchange in metal complexes with machine learning potentials
V Juraskova, G Tusha, H Zhang, L Schäfer,* F Duarte.*Faraday Discuss., 2025,256, 156, ChemRxiv 2024 Preprint.
-
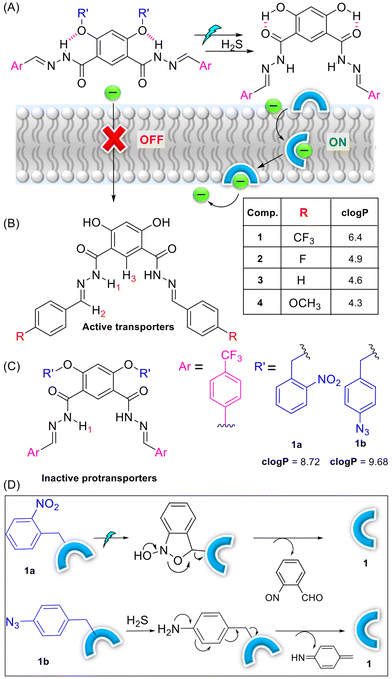
83. Stimuli-responsive anion transport utilising caged hydrazone-based anionophores
M. Ahmad, M. Flerin, H. M. Tay, A. L. Thompson, F. Duarte,* M. J. Langton.* Nanoscale 2024, 16 (46), 21545-21553
-

82. metallicious: Automated force-field parametrization of covalently bound metals for supramolecular structures
T. K. Piskorz, B. Lee, S. Zhan, F. Duarte.* J. Chem. Theory Comput. 2024, 20, 20, 9060–9071. ChemRxiv 2024 Preprint
-

81. Modeling Chemical Processes in Explicit Solvents with Machine Learning Potentials
H. Zhang, V. Juraskova, F. Duarte.* Nat. Commun., 2024, 15, 6114. ChemRxiv 2023
-

80. Origins of High-Activity Cage-Catalyzed Michael Addition
P. J. Boaler, T. K. Piskorz, L. E. Bickerton, J. Wang, F. Duarte,* G. C. Lloyd-Jones,* P. J. Lusby.* J. Am. Chem. Soc., 2024, 146, 28, 19317
-

79. Bimolecular Sandwich Aggregates of Porphyrin Nanorings
H. Gotfredsen*, J. Hergenhahn, F. Duarte. T. D. W. Claridge, H. L. Anderson*, J. Am. Chem. Soc. 2024, 146, 36, 25232–25244
-

78. PythiaCHEM : a user-friendly machine learning toolkit for chemistry
Stamatia Zavitsanou, Zonghua Bo, Emanuele Casali, Matthew Langton, and Fernanda Duarte*, ChemRxiv 2024 Preprint
-

77. CageCavityCalc (C3): A computational tool for calculating and visualizing cavities in Molecular Cages
V. Martí-Centelles,* T. K. Piskorz, F. Duarte* J. Chem. Inf. Model. 2024, 64, 14, 5604. ChemRxiv 2024 Preprint.
-

76. Responsive Anionophores with AND Logic Multi‐stimuli activation
M. Ahmad, TG Johnson, M. Flerin, F. Duarte,* M. J. Langton*, Angew. Chem. Int. Ed. 2024, e202403314
-

75. Beyond Strain Release: Delocalization-Enabled Organic Reactivity
A. J. Sterling,* R. C. Smith, E. A. Anderson,* F. Duarte, J. Org. Chem., 2024, 89, 14, 9979.
-

74. α-Amino bicycloalkylation through organophotoredox catalysis
J. Nugent, A. López-Francé, A. J. Sterling, M. Y. Tay, N. Frank, J. J. Mousseauc, F. Duarte,* E. A. Anderson. Chem. Sci., 2024, 15, 10918.
-

73. Template-Directed Synthesis of Strained meso-meso-Linked Porphyrin Nanorings
J. Van Raden, J. Deng, H. Gotfredsen, J. Hergenhahn, M. Clarke, M. Edmondson, J. Hart, J. O'Shea, F. Duarte, A. Saywell, H. L. Anderson*, Angew. Chem. Int. Ed. 2024, 63, e202400103.
-

72. Taming non-classical carbocations to control small ring reactivity
-

71. Mutate and Conjugate: A Method to Enable Rapid In-Cell Target Validation
A. M. Thomas, M. Serafini, E. K. Grant, E. A. J. Coombs, J. P. Bluck, M. Schiedel, M. A. McDonough, J. K. Reynolds, B. Lee, M. Platt, V. Sharlandjieva, P. C. Biggin, F. Duarte, T. A. Milne, J. T. Bush and S. J. Conway, ACS Chem. Biol., 2023, 18, 11, 2405–2417.
-

70. Mass spectrometric assays monitoring the deubiquitinase activity of the SARS-CoV-2 papain-like protease inform on the basis of substrate selectivity and have utility for substrate identification
1L. Brewitz, H. T. Henry Chan, P. Lukacik, C. Strain-Damerell, M. A. Walsh, F. Duarte* and C. J. Schofield,* Bioorg. Med. Chem. 2023, 95, 117498.
-

69. Picking the Lock of Coordination Cage Catalysis
-

68. Redox Reorganization: Aluminium Promoted 1,5-Hydride Shifts Allow the Controlled Synthesis of Multisubstituted Cyclohexenes
L. B. Smith, R. J. Armstrong, J. Hou, E. Smith, Ming Sze, A. J. Sterling, A. Smith, F. Duarte,* T. J. Donohoe.* Angew. Chem. Int. Ed. 2023, e202307424.
-

67. Studies on the selectivity of the SARS-CoV-2 papain-like protease reveal the importance of the P2' proline of the viral polyprotein
-

66. Dynamical nonequilibrium molecular dynamics simulations identify allosteric sites and positions associated with drug resistance in the SARS-CoV-2 main protease
H. T. H. Chan, A. S. F. Oliveira, C. J. Schofield, A. J. Mulholland,* F. Duarte,* JACS Au 2023, 3, 6, 1767–1774. BioRxiv 2022 Preprint.
-

65. Mobile Molecules: Reactivity Profiling Guides Faster Movement on a Cysteine Track
Z. Bo, Z. H. Lim, F. Duarte,* H. Bayley,* Y. Qing,* Angew. Chem. Int. Ed. 2023, e20
-

64. Visible Light Photoredox-Catalyzed Decarboxylative Alkylation of 3-Aryl-Oxetanes and Azetidines via Benzylic Tertiary Radicals and Implications of Benzylic Radical Stability
M. A. Dubois, J. J. Rojas, A. J. Sterling, H. C. Broderick, M. A. Smith, A. J. P. White, P. W. Miller, C. Choi, J. J. Mousseau, F. Duarte,* J. A. Bull,* J. Org. Chem. 2023, 88, 6476–6488.
-

63. Catalytic Enantioselective Nucleophilic Desymmetrisation of Phosphonate Esters
M. Formica , T. Rogova , H. Shi , N. Sahara , A. J. M. Farley , K. E. Christensen, F. Duarte* & D. J. Dixon,* Nat. Chem. 2023, 15, 714
-

62. Reaction dynamics of Diels-Alder reactions from machine learned potentials
T. A. Young, T. Johnston-Wood, H. Zhang, F. Duarte*, Phys. Chem. Chem. Phys. 2022, 24, 20820. ChemRxiv 2022 Preprint.
-

61. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane
N. Frank, J. Nugent, B. Shire, H. Pickford, P. Rabe, A. Sterling, T. Zarganes-Tzitzikas, T. Grimes, A. Thompson, R. Smith, C. Schofield, P. Brennan, F. Duarte and E. Anderson, Nature 2022, 611, 721. ChemRxiv 2022 Preprint.
-

60. Collective Synthesis of Illudalane Sesquiterpenes via Cascade Inverse Electron Demand (4 + 2) Cycloadditions of Thiophene S,S-Dioxides
K. H. K. Park, N. Frank, F. Duarte,* E. Anderson.* J. Am. Chem. Soc., 2022, 144, 10017–10024.
-

59. Computational Modeling of Supramolecular Metallo-organic Cages – Challenges and Opportunities
T. K. Piskorz, V. Martí-Centelles, T. A. Young, P. J. Lusby*, F. Duarte*, ACS Catal. 2022, 12, 5806–5826
-

58. Identification of Histone Peptide Binding Specificity and Small-Molecule Ligands for the TRIM33α and TRIM33β Bromodomains
A. R. Sekirnik, J. K. Reynolds, L. See, J. P. Bluck, A. R. Scorah, C. Tallant, B. Lee, K. B. Leszczynska, R. L. Grimley, R. I. Storer, M. Malattia, S. Crespillo, S. Caria, S. Duclos, E. M. Hammond, S. Knapp, G. M. Morris, F. Duarte, P. C. Biggin, S. J. Conway, ACS Chem. Biol. 2022, 17, 2753–2768. ChemRxiv 2022 Preprint.
-

57. Bending a Photonic Wire into a Ring
H. Gotfredsen, J-R Deng, J. Van Raden,M. Righetto, J. Hergenhahn, M. Clarke,A. Bellamy-Carter, J. Hart, J. O’Shea, T. D. W. Claridge, F. Duarte, A. Saywell, L. M. Herz, H. L. Anderson.*, Nat. Chem. 2022. ChemRxiv 2021 Preprint.
-

56. Amino-oxetanes as amide isosteres by an alternative defluorosulfonylative coupling of sulfonyl fluorides
J. J. Rojas, R. A. Croft, A. J. Sterling, E. L. Briggs, D. Antermite, D. C. Schmitt, L. Blagojevic, P.Haycock, A. J. P. White, F. Duarte, C. Choi, J. J Mousseau, J. A Bull, Nat. Chem. 2022, 14, 160-169.
-

55. Electrophilic Activation of [1.1.1] Propellane for the Synthesis of Nitrogen‐Substituted bicyclo[1.1.1]pentanes
S. Livesley, A. J. Sterling, C. M. Robertson, W. R. F. Goundry, J. A. Morris, F. Duarte,* C. Aïssa,* Angew. Chem. Int. Ed. 2022, 60, 4266.
-

54. Red-shifted tetra-ortho-halo-azobenzenes for photo-regulated transmembrane anion transport
A. Kerckhoffs, Z. Bo, S. Penty, F. Duarte,* M. Langton,* Org. Biomol. Chem. 2021, 19, 9058.
-

53. A Transferable Active-Learning Strategy for Reactive Molecular Force Fields
T. A. Young, T. Johnston-Wood, V. Deringer,* F. Duarte,* Chem. Sci. 2021, 12, 10944. ChemRxiv 2021 Preprint.
-

52. Discovery of SARS-CoV-2 Mpro Peptide Inhibitors from Modelling Substrate and Ligand Binding
H. T. H. Chan, M. A. Moesser, R. K. Walters, T. R. Malla, R. M. Twidale, Tobias John, H. M. Deeks, T. Johnston-Wood, V. Mikhailov, R. B. Sessions, W. Dawson, E. Salah, P. Lukacik, C. Strain-Damerell, C. D. Owen, T. Nakajima, K. Świderek, A. Lodola, V. Moliner, D.R. Glowacki, M. A. Walsh, C. J. Schofield,* L. Genovese,* D. K. Shoemark,* A. J. Mulholland,* F. Duarte,* G. M. Morris,* Chem. Sci. 2021, 12, 13686. BioRxiv 2021
-

51. Highly Active Halogen Bonding and Chalcogen Bonding Chloride Transporters with Non‐Protonophoric Activity
L.E. Bickerton, A. Docker, A.J. Sterling, H. Kuhn, F. Duarte,* P. D. Beer,* M. Langton,* Chem. Eur. J. 2021, 27, 11738.
-

50. Direct catalytic asymmetric synthesis of α-chiral bicyclo [1.1. 1] pentanes
M. L. J. Wong, A. J. Sterling, J. J Mousseau, F. Duarte,* E. A. Anderson,* Nat. Commun. 2021, 12, 1644.
-

49. Mass spectrometry reveals potential of β-lactams as SARS-CoV-2 Mpro inhibitors
T.R Malla, A. Tumber, T. John, L. Brewitz, C. Strain-Damerell, C. D. Owen, P. Lukacik, H.T.H Chan, P. Maheswaran, E. Salah, F. Duarte, H. Yang, Z. Rao, M. A. Walsh, C. J Schofield, Chem. Commun. 2021, 57, 1430.
-

48. Selectivity in organocatalysis—From qualitative to quantitative predictive models
A. J. Sterling, S. Zavitsanou, J. Ford, F. Duarte,* WIREs Comput Mol Sci. 2021, e1518.
-

47. autodE: Automated Calculation of Reaction Energy Profiles – Application to Organic and Organometallic Reactions
T. A. Young, J. J. Silcock, A. J. Sterling, F. Duarte,* Angew. Chem. Int. Ed. 2021, 60, 4266. ChemRxiv 2020.
-

46. trans-Hydroboration–oxidation products in Δ5-steroids via a hydroboration-retro-hydroboration mechanism
J. C. Hilario-Martinez, F. Murillo, J. Garcia-Mendez, E. Dzib, J. Sandoval-Ramirez, M.A Muñoz-Hernandez, S. Bernes, Laszlo Kürti, F. Duarte, G. Merino, M. A. Fernández-Herrera, Chem. Sci. 2020, 11, 12764.
-

45. Synergistic Non-Covalent Catalysis Facilitates Base-Free Michael Addition
J. Wang, T. A. Young, F.Duarte,* P. J. Lusby,* J. Am. Chem. Soc. 2020, 142, 17743.
-
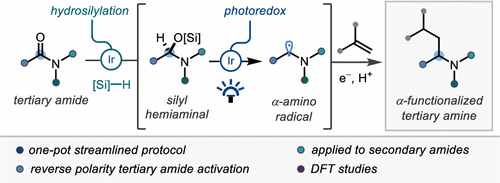
44. Reverse Polarity Reductive Functionalization of Tertiary Amides via a Dual Iridium Catalyzed Hydrosilylation & SET Strategy
T. Rogova, P. Gabriel, S. Zavitsanou, J. A. Leitch, F. Duarte,* D.J. Dixon,* ACS Catal. 2020, 10, 11438.
-

43. Harnessing Sulfinyl Nitrenes: A Unified One-Pot Synthesis of Sulfoximines and Sulfonimidamides
T. Q. Davies, M. J. Tilby, J Ren, N. A. Parker, D. Skolc, A. Hall, F. Duarte, M. C. Willis,* J. Am. Chem. Soc. 2020, 142, 15445.
-

42. Atropisomerism in diarylamines: structural requirements and mechanisms of conformational interconnection
R. Costil, A. J. Sterling, F. Duarte, J. Clayden, Angew. Chem. Int. Ed. 2020, 59 , 18670.
-

41. cgbind: A Python Module and Web App for Automated Metallocage Construction and Host-Guest Characterization
T. A. Young, R. Gheorghe, F. Duarte,* J. Chem. Inf. Model. 2020, 60, 354. ChemRxiv 2020 Preprint.
-

40. Characterization of the Zwitterionic Intermediate in 1,1-Carboboration of Alkynes
A. Bismuto, G. S. Nichol, F. Duarte,* M. J. Cowley,* and S. P. Thomas,* Angew. Chem. Int. Ed. 2020, 59, 12731.
-

39. Transmembrane anion transport mediated by halogen bonding and hydrogen bonding triazole anionophores
L.E Bickerton, A.J. Sterling, P.D. Beer, F Duarte,* M.J. Langton,* Chem. Sci. 2020, 11, 4722.
-

38. Rationalizing the diverse reactivity of [1.1.1]propellane through sigma-pi-delocalization
A.J. Sterling, A. Durr, R.C Smith, E. Anderson,* F. Duarte,* Chem. Sci. 2020, 11, 4895. ChemRxiv 2019 Preprint.
-

37. Tuning the anion binding properties of lanthanide receptors to discriminate nucleoside phosphates in a sensing array
S.H. Hewitt, G. Macey, R. Mailhot, M.R.J. Elsegood, F. Duarte, A.M. Kenwright, S. J. Butler,* Chem. Sci. 2020, 11, 3619.
-

36. Double and Triple Ionisation of Isocyanic Acid
J. H. D. Eland, R. J. Squibb, A. J. Sterling, M. Wallner, A. Hult Roos, J. Andersson, V. Axelsson, E. Johansson, A. Teichter, S. Stranges, B. Brunetti, J. M. Dyke, F. Duarte & R. Feifel, Sci. Rep. 2020, 10, 2288.
-

35. Host-Guest Induced Electron Transfer Triggers Radical-Cation Catalysis
R. L. Spicer, A. Stergiou, T. A. Young, F. Duarte,* M. D Symes,* P. J. Lusby,* J. Am. Chem. Soc. 2020, 142, 2134.
-

34. Dearomative Photocatalytic Construction of Bridged 1,3-Diazepanes
J. A. Leitch, T. Rogova, F. Duarte,* D. J. Dixon,* Angew. Chem. Int. Ed. 2020, 59, 2. ChemRxiv 2019, Preprint.
-

33. Rationalizing the Activity of an “Artificial Diels-Alderase”: Establishing Efficient and Accurate Protocols for Calculating Supramolecular Catalysis
T. A. Young, V. Martí-Centelles, J. Wang, P. J. Lusby,* F. Duarte,* J. Am. Chem. Soc. 2020, 142, 1300. ChemRxiv 2019, Preprint.
-

32. The Energetic Significance of Metallophilic Interactions
Q. Zheng, S. Borsley, G.S Nichol, F. Duarte, S. L. Cockroft, Angew. Chem. Int. Ed. 2019, 58, 12617.
-

31. Catalytic Asymmetric Synthesis of Cyclohexanes by Hydrogen Borrowing Annulations
R. Armstrong, W. Akhtar, T. Young, F. Duarte,* T. J. Donohoe,* Angew. Chem. Int. Ed. 2019, 58, 12558.
-

30. Relative Binding Energies Predict Crystallographic Binding Modes of Ethionamide Booster Lead Compounds
N. Tatum, F. Duarte, S. C. L. Kamerlin, E. Pohl, J. Phys. Chem. Lett. 2019, 10, 2244.
-

29. A General Route to Bicyclo[1.1.1] pentanes through Photoredox Catalysis
J. Nugent, C. Arroniz, B. Shire, A. J. Sterling, H. D. Pickford, M. L. J. Wong, S. J. Mansfield, D. F. J. Caputo, B. Owen, J. J. Mousseau, F. Duarte,* E. Anderson,* ACS Catal. 2019, 9, 9568. ChemRxiv 2019, Preprint.
-

28. Stereospecific 1,3-H Transfer of Indenols Proceeds via Persistent Ion-Pairs Anchored By NH···π Interactions
D. M. H. Ascough, F. Duarte, and R. S. Paton, J. Am. Chem. Soc. 2018, 140, 16740.
-

27. Host-Guest chemistry of self-assembled hemi-cage systems: The dramatic effect of lost pre-organization
V. Martí-Centelles, F. Duarte,* P. J. Lusby,* Isr. J. Chem. 2018, 58, 1.
Publications (Edinburgh, 2017-2028)
-
26. Bio-inspired Domino oxa-Michael/Diels–Alder/oxa-Michael Dimerization of para-Quinols
N. J. Green, C. A. Connolly, K. PW Rietdijk, G. S. Nichol, F. Duarte,* A. L. Lawrence,* Angew. Chem. Int. Ed.2018, 57, 6198.
-
25. Cation–π interactions in protein-ligand binding: theory and data-mining reveal different roles for lysine and arginine
K. Kumar, S. M. Woo, T. Siu, W. A. Cortopassi, F. Duarte,* and R. S. Paton,* Chem. Sci. 2018, 9, 2655.
-
23. Total Synthesis of a Dimeric Thymol Derivative Isolated from Arnica sachalinensis
De Silvestro, S. L. Drew, G. S. Nichol, F. Duarte,* A L. Lawrence.* Angew. Chem. Int. Ed. 2017, 56, 1. (HOT article)
-
24. Molecular Recognition in Asymmetric Counteranion Catalysis: Understanding Chiral Phosphate-Mediated Desymmetrization.
59. F. Duarte,* R. S. Paton.* J. Am. Chem. Soc., 2017, 139, 8886
-
22. Theory and Applications of the Empirical Valence Bond Approach: From Physical Chemistry to Chemical Biology (Book)
Fernanda Duarte and Shina Caroline Lynn Kamerlin (Editors). John Wiley & Sons, Ltd (2017)
Publications (Postdoc and PhD)
-
21. Evolutionary repurposing of a sulfatase: A new Michaelis complex leads to efficient transition state charge offset
C. M. Miton, S. Jonas, G. Fischer, F. Duarte, M. F. Mohamed, B. van Loo, B. Kintses, S. C. L. Kamerlin, N. Tokuriki, M. Hyvönen, and F. Hollfelder. Proc. Natl. Acad. Sci. U.S.A2018, 115, E7293.
-
20. Theory and Applications of the Empirical Valence Bond Approach: From Physical Chemistry to Chemical Biology (Book))
Fernanda Duarte, Anna Pabis, Shina Caroline Lynn Kamerlin. John Wiley & Sons, Ltd (2017)
-
19. Promiscuity in the Enzymatic Catalysis of Phosphate and Sulfate Transfer (Review)
A Pabis, F Duarte, SCL Kamerlin Biochemistry, 2016, 55, 22, 3061–3081
-
18. Computing Organic Stereoselectivity – from Concepts to Quantitative Calculations and Predictions. (Tutorial Review).
Q. Peng‡, F. Duarte‡, R.S. Paton. Chem. Soc. Rev. 2016 45, 6093
-
17. Mechanisms of Histone-Modifying and Reading Enzymes: the Role of the Protein Environment from a Computational Perspective
W. A. Cortopassi, K. Kumar, F. Duarte, A. S. Pimentel, R. S. Paton. J. Mol. Graph. Model. 2016, 67, 69
-
16. The Competing Mechanisms of Phosphate Monoester Dianion Hydrolysis
F. Duarte, A. Barrozo, J. Åqvist, N. H. Williams, S. C. L. Kamerlin. J. Am. Chem. Soc. 2016, 138, 1066
-
15. Recent advances in QM/MM free energy calculations using reference potentials
F. Duarte,* B.A Amrein, D. Blaha-Nelson, SCL Kamerlin.* Biochimica et Biophysica Acta (BBA)-General Subjects 2015, 1850 (5), 954-965
-
14. Expanding the Catalytic Triad in Epoxide Hydrolases and Related Enzymes
B. A. Amrein, P. Bauer, F. Duarte, Å. J. Carlsson, A. Naworyta, S. Mowbray, M. Widersten, S. C. L. Kamerlin. ACS Catalysis 2015, 5, 5702.
-
13. Cooperative Electrostatic Interactions Drive Functional Evolution in the Alkaline Phosphatase Superfamily
A. Barrozo‡, F. Duarte‡, P. Bauer, A. T. P. Carvalho, S. C. L. Kamerlin. J. Am. Chem. Soc. 2015, 137, 9061
-
12. Resolving Apparent Conflicts Between Theoretical and Experimental Models of Phosphate Monoester Hydrolysis
F. Duarte, J. Åqvist, N. H. Williams, S. C. L. Kamerlin. J. Am. Chem. Soc. 2015, 137, 1081 (Cover article and Spotlight)
-
11. Conformational and Chemical Landscapes of enzyme Catalysis (Book Chapter)
A. TP Carvalho, F. Duarte, K. Vavitsas, S. C. L. Kamerlin. Computational Approaches to Protein Dynamics: From Quantum to Coarse-Grained Methods (2014)
-
10. Empirical Valence Bond Simulations of the Hydride Transfer Step in the Monoamine Oxidase B Catalyzed Metabolism of Dopamine.
M. Repic, R. Vianello, M. Purg, F. Duarte, P. Bauer, S. C. L. Kamerlin, J. Mavri. Proteins 2014, 82, 3347
-
9. Catalytic Zinc Complexes for Phosphate Diester Hydrolysis.
E. Y. Tirel, Zoë Bellamy, H. Adams, F. Duarte, N. H. Williams. Angew. Chem. Int. Ed. 2014, 53, 8246
-
8. Force-field Independent Metal Parameters Using a Non-bonded Dummy Model
F. Duarte, P. Bauer, A. Barrozo, B. A. Amrein, M. Purg, J. Åqvist, S.C.L. Kamerlin. J. Phys. Chem. B, 2014, 118, 4351
-
7. Concerted or Stepwise: How Much Do Free-Energy Landscapes Tell Us about the Mechanisms of Elimination Reactions?
F. Duarte, S. Gronert S. C. L. Kamerlin. J. Org. Chem., 2014, 79, 1280
-
6. The Alkaline Hydrolysis of Sulfonate Esters: Challenges in Interpreting Experimental and Theoretical Data
68. F. Duarte, T. Geng, G. Marloie, A. O. Al Hussain, N. H. Williams S. C. L. Kamerlin. J. Org. Chem., 2014, 79, 1280 (Cover and Feature article)
-
5. How Does Pin1 Catalyze the Cis-Trans Prolyl Peptide Bond Isomerization? A QM/MM and Mean Reaction Force Study
E. Vöhringer-Martinez, F. Duarte, A. Toro-Labbé. J. Phys. Chem. B, 2012, 116, 12972
-
4. Modeling Catalytic Promiscuity in the Alkaline Phosphatase Superfamily
F. Duarte, B. A. Amrein S. C. L. Kamerlin. Phys. Chem. Chem. Phys., 2013, 15, 11160 (Cover and HOT article)
-
3. The Mechanism of H2 Activation by (Amino)Carbenes
1. F. Duarte, A. Toro-Labbé. J. Phys. Chem. A, 2011, 115, 3050
-
2. Insights on the Mechanism of Proton Transfer Reactions in Amino Acids
F. Duarte, E. Vöhringer-Martinez, A. Toro-Labbé. Phys. Chem. Chem. Phys., 2011, 13, 7773
-
1. Water Catalysis of the keto-enol Tautomerization Reaction of Thioformic Acid
F. Duarte, A. Toro-Labbé. Mol. Phys. 2010, 108, 1375
Empirical Valence Bond Book
-

Theory and Applications of the Empirical Valence Bond Approach: From Physical Chemistry to Chemical Biology
Description. Over the past couple of decades, Empirical Valence Bond (EVB) approaches have become one of the key tools for studying chemical processes in condensed phases and proteins. This book provides a comprehensive overview of these advances, highlighting how they have shaped our current understanding of enzyme catalysis and chemical processes in general. Written by leading scientists in the field, the book focuses on the applications and extension of the original EVB approach to a variety of different areas of research, including reaction dynamics, design of artificial catalysts, and the study of complex biological problems.
The book begins with concise yet comprehensive introduction to the basic concepts and historical background of valence bond theory, and EVB specifically. Subsequent chapters discuss the application of EVB models to a broad range of molecular systems of chemical and biological interest, and highlight the effectiveness of EVB to study chemical processes in the condensed phase and in enzymes.




